A research team headed by Dr. Assaf Zaritsky of Ben-Gurion University of the Negev has determined some of the characteristics of melanoma cells that are likely to metastasize to other parts of the body, a first and important step in developing novel treatments and eventually a cure.
Melanomas are a form of aggressive skin cancer.
The is the next step in research about which Zaritsky presented in December 2018 at the American Society for Cell Biology/EMBO conference in San Diego. He began the research with Gaudenz Danuser of the University of Texas Southwestern Medical Center at Dallas during his postdoctoral research.
Using deep neural networks – sophisticated mathematical modeling to process data in complex ways – Zaritsky’s team created a representation of the functional state of individual cells that can help predict the chances that a stage III melanoma will progress to stage IV, the most advanced phase of melanoma and a serious form of skin cancer. This means that the cancer has spread from the lymph nodes to other remote organs.
“The dream is that a person would come with stage III melanoma and doctors could predict if it would progress to stage IV or not and, based on that, adjust his or her treatment,” he told The Jerusalem Post.
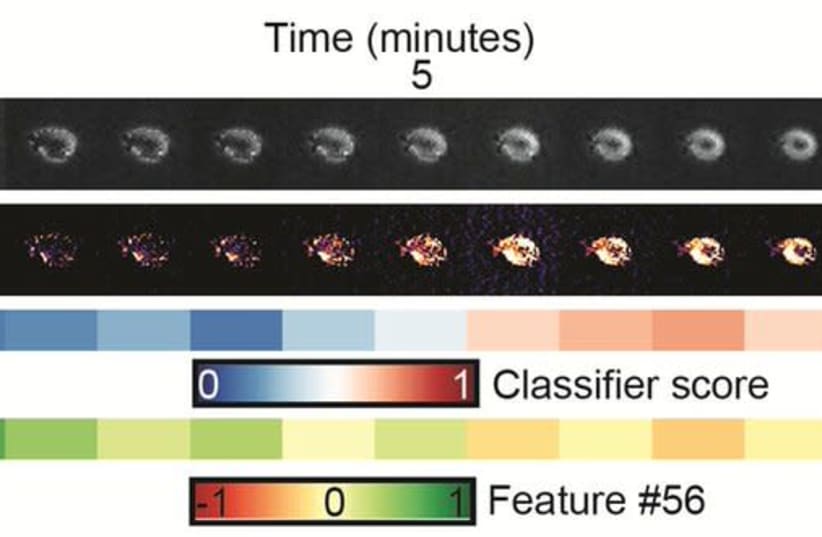
By computationally generating cell images that have never been observed experimentally and by exploiting temporal information from live cell imaging experiments, the team reverse engineered the physical properties of the hidden image information that discriminates melanoma cells with low-versus-high metastatic efficiency. This revealed that cells that are likely to metastasize have pseudopodial extensions or miniature protrusions as well as increased light scattering.
“Deep neural network machine learning is a very powerful tool and can identify hidden patterns in complex cell imaging data that we do not see with our eyes,” Zaritsky explained. However, he said that these machine learning techniques are often criticized as uninterpretable black boxes, lacking the ability to provide meaningful explanations for the cell properties that drive the machine’s prediction.
His team used melanoma cells from patients that were previously implanted into mice and showed associated metastatic potential to the patient’s outcome. The team investigated whether this potential can be predicted from patterns in the cells’ appearance.
Normally, metastatic progression is predicted through a combination of genetic tests and patient history and static histological slides, which would not give information about the changes happening within the cells.The first stage of his research allows doctors to predict what is likely to happen to the melanoma cancer cells and treat accordingly. This newly revealed second stage of the research helps identify what are the properties of these cells that metastasize, “so we can create new treatments and eventually cures… If we know the properties of the cell that is going to metastasize, we can look for drugs.”
He said that, “We would take cells from patients and apply different drugs to them to see how these drugs change the cells – whether they behave more like cells with less metastatic potential.”
Zaritsky stressed that his work is “a long way from a cure, but now we can start thinking about it much more than we could before.”
Additional researchers include Andrew Jamieson, Erik Welf and Andres Nevarez in Texas. The research was already published on bioRxiv.org and has been submitted for evaluation in a peer review journal.