Breakthrough Israeli technology for treating acute migraine headaches has been found to be more effective than standard-care medications for adolescents, according to a new study published in the Oxford Journal of the American Academy of Pain Medicine.
Nine percent of children and adolescents worldwide suffer from migraines. The affliction has been associated with poor academic performance, reduced school attendance and as having a negative impact on social interaction and quality of life.
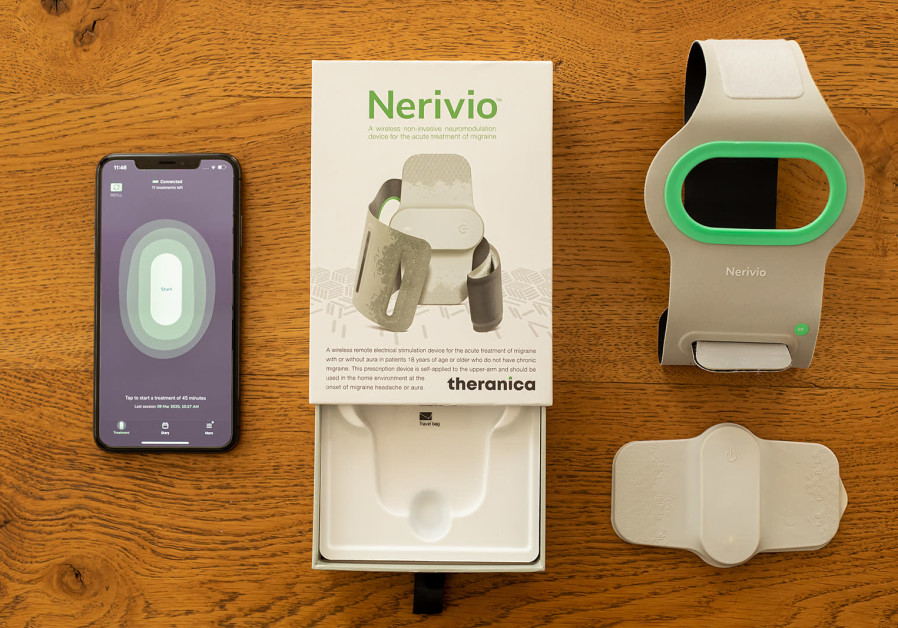
Nerivio, a remote electrical neuromodulation (REN) device developed by Netanya-based Theranica, activates the body’s native conditioned pain modulation mechanism to treat headache and other symptoms associated with migraine by stimulating the free nerve endings in the upper arm.
At two hours post-treatment, pain freedom was achieved by 37% of participants with REN compared to 9% of the participants who took oral triptans and over-the-counter analgesic medications.
In addition, pain relief was achieved by 71% with REN versus 57% with medications, consistency of pain freedom was achieved by 40% with REN versus 9% and consistency of pain relief was achieved by 80% with REN versus 57%.
Moreover, REN has no side effects, studies have shown. In the adolescent study, only one participant reported a mild device-related adverse event – a temporary feeling of pain in the arm, which was resolved after the treatment without requiring intervention.
Those who take triptans often report drowsiness, fatigue and difficulty concentrating for up to 24 hours after taking a pill.
The study included 35 adolescents who were treated in two phases. In the run-in phase, migraine attacks were treated with medications. In the intervention phase, they were treated with REN.
An additional, large-scale, blinded comparative effectiveness and tolerability study would still be needed to confirm results. However, previous studies have shown similar effectiveness in adults.
THE DEVICE is Food and Drug Administration approved in the United States and approved for use by the Health Ministry in Israel, where it is available through one's health fund.
Nerivio has treated more than 100,000 migraines in over 14,000 patients, the company said. It is treating about 100 patients every month in Israel.
It was developed with research and guidance from Prof. David Yarnitsky, head of the Neurology Department at the Technion – the Israel Institute of Technology. The company’s CEO, Alon Ironi, an electronics engineer, decided to develop the device when his daughter began to suffer from migraines.
Migraines are one of the most common and debilitating conditions in the world. The headaches often render those who get them unable to work and negatively affect productivity.
"Migraine is the third most prevalent disorder in the world and affects approximately one billion people,” said James Johnson, managing director, MedTech Breakthrough.
Nerivio won the “Best New Technology Solution” award in the Pain Management category at the MedTech Breakthrough Awards in May. There were 3,850 nominations for the award from 17 countries.
Ironi walked The Jerusalem Post through a standard treatment, explaining that users turn the device on at the onset of an attack – either a headache, or other related migraine symptoms, such as vision deficiency and nausea, and hypersensitivity to sound, light or smell.
Patients place the device on their upper arms and start the treatment, which is meant to take 45 minutes, but can take less time.
“During treatment, people can continue with their normal activities,” Ironi said. “The only two things they cannot do are swim, or take a shower since this is an electronic device.”
When the session is over, patients simply return the device to its box and store it for the next attack.
Nerivio comes with an application that makes the treatment more personalized, he said. An interactive migraine diary tracks treatment sessions and symptoms that can then be shared with one’s doctor. The device also provides recommendations for how to optimize treatments for better personal results.
“There is nothing like it out on the market for migraines,” said Dr. Shira Markowitz, an independent general neurologist and a headache and facial pain specialist with Shaare Zedek Medical Center and Maccabi Health Services.
She told the Post that “what is fascinating is that it does not give stimulation to the nerves around the cranium that are generally thought to be involved in migraines.” Rather, “it is sort of like when something hurts and we punch ourselves in another place – and then our body forgets the other pain or releases chemicals that cause pain relief.”
Technion UK CEO Alan Aziz added that “Nerivio could help keep future migraines at arm’s length.”
