Not all blood vessels are created equal. Those that supply different organs vary significantly from one to another. For example, because the kidneys engage in filtration, their blood vessel walls have small holes that make possible the efficient passage of substances. In the brain, the same walls are nearly sealed, ensuring a protective blockage known as the blood-brain barrier. Blood vessel walls in the lungs are suited to facilitating the exchange of gases.
Just as our family origins tend to shape our future in many ways, the same thing holds for vessels that carry blood through the body. Researchers at the Weizmann Institute of Science in Rehovot has just published in the prestigious journal Nature under the title “Generation of specialized blood vessels via lymphatic transdifferentiation” that blood vessels forming from unexpected progenitors show that this unusual origin determines the vessels’ future function.
“We found that blood vessels must derive from the right source so as to function properly – it’s as if they remember where they came from,” said team leader Prof. Karina Yaniv.
"It’s as if [blood vessels] remember where they came from.”
Professor Karina Yaniv
Despite the vital importance of the vascular system, how such differences between various blood vessels come has not been well understood by scientists. Until now, these vessels were known to originate from two sources – existing blood vessels or progenitor cells that mature and differentiate to form the vessel walls. In the new study, postdoctoral fellow Dr. Rudra Das, working in Yaniv’s lab in the immunology and regenerative biology department, discovered that blood vessels can develop from a previously unknown source – lymphatic vessels. This third type was revealed in transgenic zebrafish, a type of minnow, whose cells were labeled with newly established fluorescent markers that enable tracing.
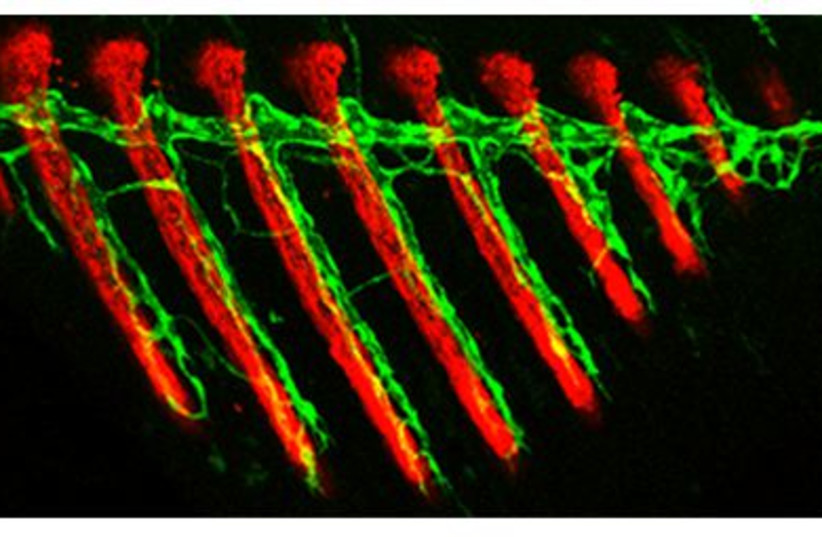
Because of its fully sequenced genome, easy genetic manipulation, high fertility, external fertilization, rapid development and its nearly transparent embryos, zebrafish are a unique model animal for biomedical research, including studies of biological processes and human diseases.
“It was known that blood vessels can give rise to lymphatic vessels, but we’ve shown for the first time that the reverse process can also take place in the course of normal development and growth,” Das explained. By tracing the growth of fins on the body of a juvenile zebrafish, Das saw that even before the bones had formed, the first structures to emerge in a fin were lymphatic vessels. Some of these vessels then lost their characteristic features, transforming themselves into blood vessels.
THIS SEEMED very wasteful. Why, asked the researchers, hadn’t the blood vessels in the fins simply sprouted from a large nearby blood vessel? Das and colleagues provided an explanation by analyzing mutant zebrafish that lacked lymphatic vessels. They found that when such vessels were absent, the blood vessels did sprout in the growing fins of these mutants by branching from existing ones nearby.

Surprisingly however, in this case, the fins grew abnormally, with malformed bones and internal hemorrhaging. A comparison revealed that in the mutant fish, excessive numbers of red blood cells entered the newly formed blood vessels in the fins, while in regular fish with lymphatic-derived blood vessels, this entry was controlled and restricted.
The scarcity of red blood cells apparently created low-oxygen conditions known to benefit well-ordered bone development. In the mutant fish, on the other hand, an excess of red blood cells disrupted these conditions, which could well explain the observed abnormalities. In other words, only those blood vessels that had matured from lymphatic vessels were perfectly suited to their specialized function – in this case, proper fin development.
Regenerating organs
Since zebrafish, unlike mammals, exhibit a remarkable capacity for regenerating most of their organs, Das and colleagues set out to explore how a fin would regrow following injury. They saw that the entire process they had observed during the fin’s development repeated itself during its regeneration – namely, lymphatic vessels grew first, and only later did they transform into blood vessels. “This finding supports the idea that creating blood vessels from different cell types is no accident – it serves the body’s needs,” Das says.
The study’s findings are likely to be relevant to vertebrates other than zebrafish, including humans. “In past studies, whatever we discovered in fish was usually shown to be true for mammals as well,” Yaniv added. “On a more general level, we’ve demonstrated a link between the ‘biography’ of a blood vessel cell and its function in the adult organism. We’ve shown that a cell’s identity is shaped not only by its place of ‘residence’ or the kinds of signals it receives from surrounding tissue but also by the identity of its ‘parents.’”
Yaniv, whose lab specializes in studying the lymphatic system, feels particularly vindicated by the new role the study has revealed for lymphatic vessels: “They are usually seen as poor cousins of blood vessels, but perhaps it’s just the opposite. They might actually take precedence in many cases.”
Study participants also included Yaara Tevet, Stav Safriel, Noga Moshe, Giuseppina Lambiase, Dr. Ivan Bassi, Dr. Julian Nicenboim and Dr. Roi Avraham of Weizmann’s immunology and regenerative biology department; Dr. Yanchao Han and Prof. Kenneth Poss of Duke University School of Medicine in North Carolina; Matthias Brückner and Prof. Wiebke Herzog of the Friedrich-Alexander Universität Erlangen-Nürnberg and Max Planck Institute for Molecular Biomedicine; and Dana Hirsch and Dr. Raya Eilam-Altstadter of Weizmann’s veterinary resources department.
