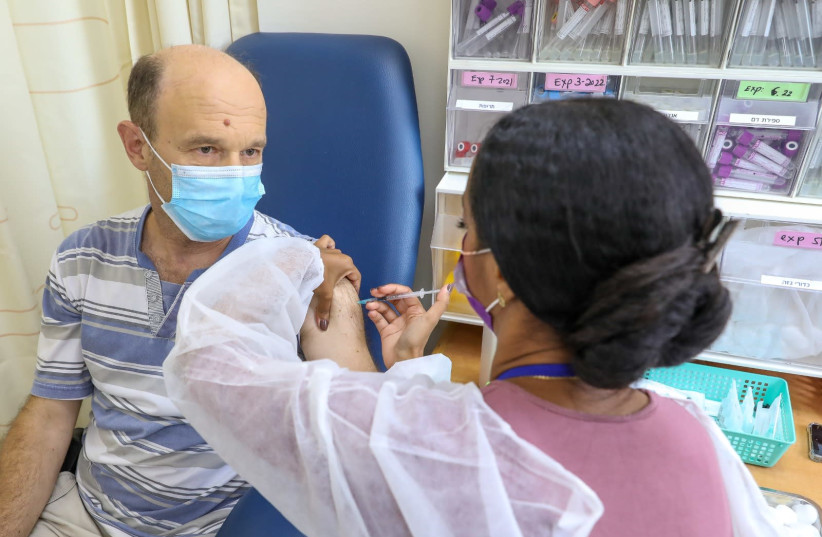
Researchers at Mount Sinai's Icahn School of Medicine in New York have developed a rapid blood test that measures the intensity and length of a person's immunity to SARS-CoV-2, the virus that causes COVID-19.
According to a study published in the peer-reviewed Nature Biotechnology this month, the development of the test, which provides results within 24 hours, will allow widespread monitoring of the population's immunity to the virus, in addition to insight on vaccine effectiveness.
T cell response vs antibodies
Researchers explained that the test measures the activation of T cells, which are part of the human immune response to a COVID infection or vaccination and help fight severe illness or death. Both T cells and antibodies lead to long-term protection from infection. Although harder to evaluate, T cell memory represents a fundamental factor in the immunity against the virus and may be able to ensure protection after the antibodies are gone, according to experts. This is one of the first studies to successfully measure T cell responses because of the associated technical challenges.
The team focused to the two assays that gave the most scalability. One, the qTACT assay, was reported to be "accurate and sensitive but had a relatively longer processing time of 24 hours per 200 blood samples, a moderate price, and a medium level of technical skill." The other, the dqTACT assay, was "accurate and had a reduced processing time and cost, and required minimal lab experience, making it easy to implement," according to the study.
"These T cells have an important role in protection from emerging mutant strains, thus immediately gauging the impact that viral mutations might have on cellular immunity.”
Jordi Ochando
“The assays presented here are based on the ability of SARS-CoV-2 T cells to respond to peptides covering different proteins of the virus,” said senior author, Jordi Ochando, PhD, Assistant Professor of Oncological Sciences at the Tisch Cancer Institute at Mount Sinai and Assistant Professor of Medicine (Nephrology), and Pathology, Molecular, and Cell-Based Medicine at the Icahn School of Medicine at Mount Sinai. “With the possibility of using different peptide pools, our approach represents a flexible strategy that can be easily implemented to detect the presence of T cells responding to different viral proteins. These T cells have an important role in protection from emerging mutant strains, thus immediately gauging the impact that viral mutations might have on cellular immunity.”
Megan Schwarz, a graduate student at Mount Sinai and first author of the study, added, “Precise measurement of cellular responses underlying virus protection represents a crucial parameter of our levels of immune defense.”
