Ramot, the technology transfer company of Tel Aviv University (TAU) has signed a collaboration with the Bayer pharmaceutical company to test new drugs on 3D-printed heart tissues, a new, more efficient method of testing.
Prior to reaching the pharmaceutical shelves, drugs must undergo a complicated process of trials and screenings which include testing on human tissue cultures in petri dishes, and are often tested on animals before finally being approved for human clinical trials. The new method of testing on 3D-printed hearts bypasses many of these screening methods, making it faster, cheaper and more efficient.
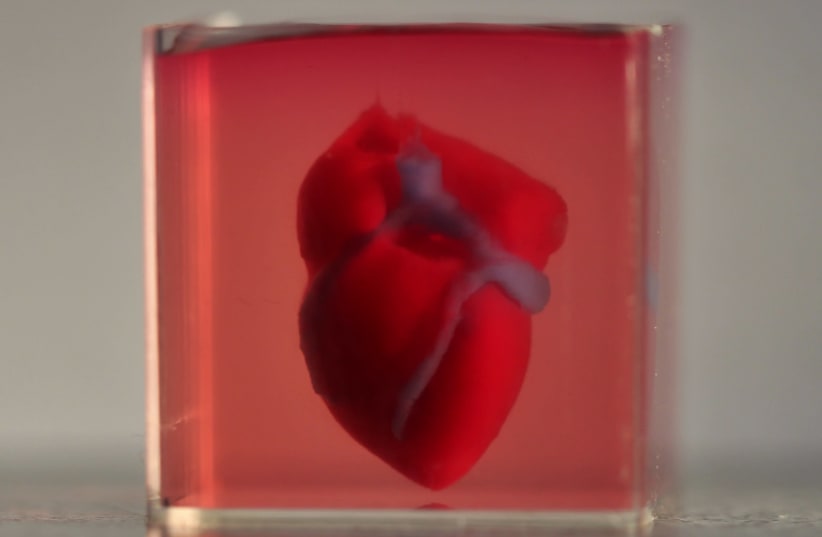
“In a petri dish, all the cells line up in 2D, and it’s only one type of cell” said Prof. Tal Dvir of TAU, the scientist responsible for developing the world's first working 3D printed heart. “In contrast, our engineered tissues are 3D-printed, and therefore better resemble real heart tissues," she added.
According to the collaboration agreement, Bayer and TAU will collaborate to develop and validate a platform for in vitro cardiotoxicity screening in Dvir’s Laboratory for Tissue Engineering and Regenerative Medicine. In short this means that the new pharmaceuticals will be tested on human heart tissue printed in the laboratory.
"Our printed tissues contain cardiac muscle, blood vessels and the extracellular matrix which connects the different cells biochemically, mechanically and electrically. Moving away from petri dishes to 3D-printed tissues could significantly improve drug tests, saving precious time and money with the hope of producing safer and more effective medication,” Dvir said.
In April of last year, Dvir's team was responsible for printing the world's first 3D vascularized, engineered heart, which was made using a patient’s own cells and biological material. Until then, scientists had only printed simple tissues without blood vessels.
In years to come, Dvir’s team and Bayer plan to test new medications for toxicity and efficacy using printed, whole human hearts.
“Our end goal is to engineer whole human hearts, including all the different chambers, valves, arteries and veins – the best analogue of this complex organ – for an even better toxicological screening process.”
Additionally, researchers estimate that it will be possible to print personalized organs and tissues within 10-15 years. With this ability, the need for organ donations will be eliminated along with the risk of transplant rejection. To make further use of the application, Ramot licensed the technology to a spin-off company called Matricelf, which focuses on engineering personalized spinal-cord implants to treat paralyzed patients.