A breakthrough technology for growing tissue for transplantation by printing it into a microgel bath as support material was developed by the Technion.
Tissue printing is an innovative approach for creating tissue for transplantation. In this technique, also called bio-printing, living cells are embedded in biological ink and printed layer upon layer. The printed tissue then undergoes growth for days or weeks until it is ready for printing.
The peer-reviewed research, published in Advanced Science, was led by Professor Shulamit Levenberg and her doctoral student Majd Machour from the Faculty of Biomedical Engineering along with Professor Havazelet Bianco-Peled and doctoral student Noy Hen from the Wolfson Faculty of Chemical Engineering and The Norman Seiden Multidisciplinary Graduate program in Nanoscience & Nanotechnology.
“many research groups around the world are working on improving tissue printing, but most of them are focusing on the printing phase and the initial product – the printed tissue. However, the growth phase of the tissue – that is, the period between the printing and the transplantation in the target organ – is no less important," Prof. Levenberg explained.
“many research groups around the world are working on improving tissue printing, but most of them are focusing on the printing phase and the initial product – the printed tissue. However, the growth phase of the tissue – that is, the period between the printing and the transplantation in the target organ – is no less important."
Professor Shulamit Levenberg
"This is a complex period in which the printed cells divide, migrate, and secrete their extra cellular matrix and attach to each other to create the tissue. One of the problems is that in this complex process, the tissues tend to distort and shrink in an uncontrolled manner.”
A major challenge in tissue engineering is fabricating 3D constructs with structural and functional properties that mimic the 3D environment of living tissue in a reproducible and stable manner. Extrusion-based bioprinting emerged as a valuable technique to fabricate cell-containing 3D scaffolds with high complexity and accuracy, according to the study.
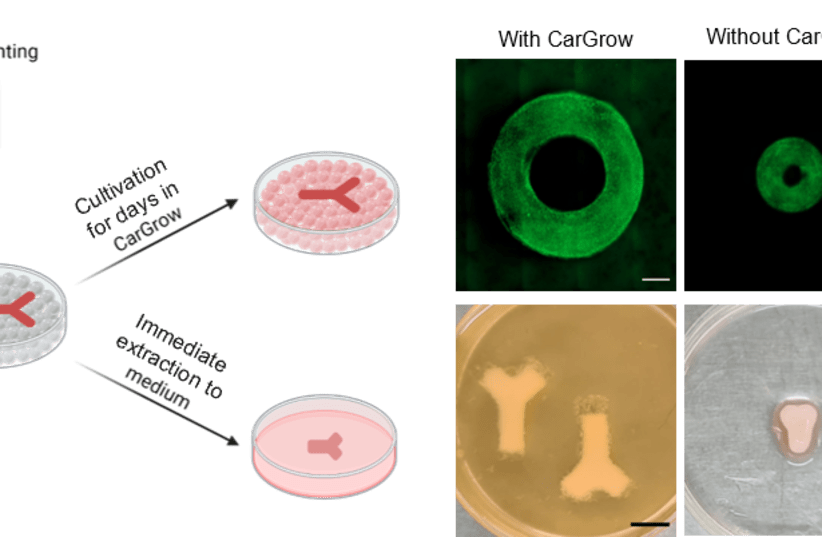
The researchers developed methods for preventing the uneven shrinkage of the printed tissue in the weeks after printing. The solution was found through changing the medium in which the tissue is printed and grown.
The new concept, print-and-grow, is based on an original concept developed by the researchers – a microgel used as a support material in the process, CarGrow, which is a substance mainly composed of carrageenan (Carrageenan-K) and is produced from red algae.
Thus the new support bath preserves the size of the tissue after printing and prevents it from shrinking and losing its shape.
This process allows reliable and controlled production of functional tissue in the desired size and shape. Since this material is transparent, it makes it possible for the scientists to monitor the development of the tissue through imaging.
A step-by-step of bio-printing
The support material allows for free movement of the nozzle needle during printing while maintaining the structure by trapping the extruded bioink between the particles upon removing the stress.
Next, the printed structure is cured, followed by removing the support material using an external trigger such as temperature change, enzymatic cleavage, or mechanical force.
Finally, the printed construct is transferred into a liquid medium for tissue maturation and growth. Direct printing within support materials can provide mechanical support to low viscosity biomaterials, which are compatible with living cells.