By now, thanks to Prof. Shulamit Levenberg at the Technion-Israel Institute of Technology’s stem cell and tissue engineering laboratory, it’s no longer big news that real muscle, fat and a vascular-like system similar to a ribeye steak can be 3D bioprinted using cells from a cow rather than slaughtering an animal. Aleph Farms, where Levenberg is chief scientific adviser, made top headlines last year when it presented the world’s first cultivated ribeye steak.
But now, this world leader in tissue engineering and her team at the Haifa institute can boast about a new accomplishment – creating edible muscle tissue by bioprinting a plant-based scaffold and living animal cells.
Levenberg and doctoral student Iris Ianovici of the Technion’s Faculty of Biomedical Engineering worked in collaboration with the cultivated meat producing company that the professor helped establish along with Dr. Yedidya Zagury, Dr. Idan Redensky and Dr. Neta Lavon.
They just published their study in the journal Biomaterials under the title “3D-printable plant protein-enriched scaffolds for cultivated meat development.”
This new technology of cultured or cell-based meat is not only a scientific and engineering accomplishment; the researchers predict that in the near future, it will make possible the large-scale production of cultivated meat without requiring the raising and suffering of animals in slaughterhouses and the environmental damage from methane gas that cows release into the atmosphere.
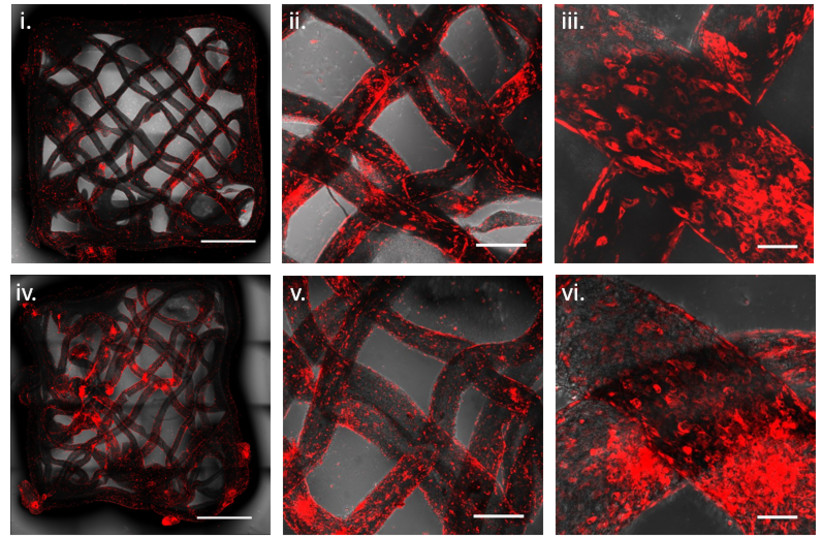
To meet the expectations of meat lovers, technologies are necessary that allow for the production of whole muscle cuts that are as similar as possible in terms of taste, smell, and culture.
Recently, a Korean team headed by H.W. Kang demonstrated 3D-printing of bovine satellite cells and adipose-derived stem cells but the materials used were either animal-derived or non-edible.
The ability to produce a wide variety of cultivated meat products was the main focus for creating thicker cultivated steaks while using alternative materials as scaffolds that support cell adherence and proliferation.
In the article, the researchers presented a solution to these challenges by using an alternative bio-ink to print scaffolds from animal-free proteins, as well as living animal cells. The bio-ink contains the cells that will form the muscle tissue – satellite cells originating from a biopsy taken from livestock and formulated by combining alginate, a compound found within the cell walls of brown algae – and proteins isolated from soy or pea proteins.
The printing process, which enables the creation of protein-enriched scaffolds with different geometries, is based on a method in which the bio-ink is deposited into a suspension bath that supports the materials during printing.
The cells successfully matured to create muscle fibers as the tissue grew. Since the geometry of the scaffold can be controlled, it’s possible to control the introduction of nutrients and the removal of waste from the developing tissue.
“In the engineering process we developed, we tried to mimic the natural process of tissue formation inside the animal’s body as much as possible,” said Levenberg. “The cells successfully adhered to the plant-based scaffold, and the growth and differentiation of the cells proved successful as well. Our bio-ink led to a consistent distribution of the cells across the bioprinted scaffold, promoting growth of the cells on top of it.
"Since we used non-animal-derived materials like non-allergenic pea protein, our findings promise greater development of the cultivated meat market moving forward.”
