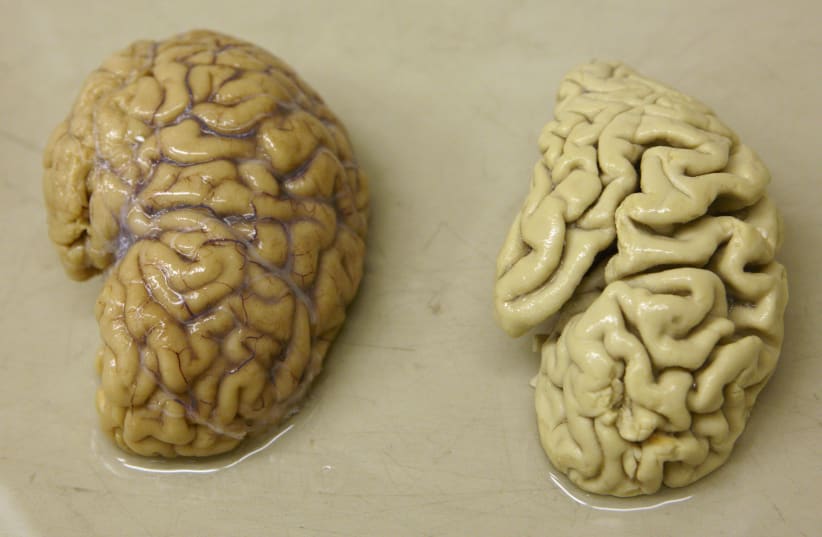
A potential treatment for Alzheimer’s disease and reversal of some of the neurodegeneration caused by the disease has been discovered by Neuroscientists from the Massachusetts Institute of Technology (MIT).
The new treatment has been detailed in a peer-reviewed study, which has been published on Wednesday in the journal PNAS.
The new treatment, using peptides, interacts with the CDK5 enzyme. The CDK5 enzyme is overactive in Alzheimer’s patient’s brains. Fortunately, the peptide has not shown signs of reacting with the essential CDK1 enzyme which shares structural similarities with CDK5.
What is CDK5 and how does the treatment work?
CDK5 binds with P35 and changes the cellular structure. The change allows P35 to Phosphorylate and become P25. P25 has a longer half-life than P35.
CDK5 can bind to P25, and the combination can target the Tau protein and add Phosphorylate, which then causes the neurofibrillary tangles associated with Alzheimer’s disease, Parkinson’s disease and frontotemporal dementia.
The new treatment has undergone animal trials and shown promising results. Researchers treated mice with the peptide and the peptide caused massive reductions in neurodegeneration and DNA damage in the brain.
After the treatment, the mice showed reduced mutations of Tau pathologies and neuron loss. The researchers also observed behavioral improvements, with the treated mice performing better in maze tests that relied on spatial memory.
“We found that the effect of this peptide is just remarkable,” said Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory and the senior author of the study to MIT News. “We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
The researchers were also able to see that the peptide was able to penetrate the blood-brain barrier and reach the neurons in the hippocampus.
“We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
Li-Huei Tsai
The injected peptide also resulted in the expression of 20 genes, which are typically activated by the gene regulators MEF2. These genes can be resilient to cognitive impairments in the brains of people with TAU tangles.
“Further development of such peptide inhibitors toward a lead therapeutic candidate, if proven to be selective for the target and relatively free of clinical side effects, may eventually lead to novel treatments for neurodegenerative disorders ranging from Alzheimer’s disease to Frontotemporal dementia to Parkinson’s disease,” says Stuart Lipton, a professor of neuroscience at Scripps Research in an interview with MIT News.
Other treatments use molecular treatment which is accompanied by a strong host of side effects. However, using a peptide with an identical CDK5 sequence, the scientists were able to reduce the burden of treatment.
The peptide used also is 12 amino acids long, which is longer than other peptide drugs which are 5-10 amino acids long.
“From a peptide drug point of view, usually smaller is better,” Tsai told MIT News. “Our peptide is almost within that ideal molecular size.”
The researchers plan to carry out further testing to see which forms of dementia might benefit from this treatment. They plan to test the new treatment for HIV-induced dementia, diabetes-linked cognitive impairment and frontotemporal dementia.
“It’s very hard to say precisely which disease will most benefit, so I think a lot more work is needed,” she said to MIT News.